About Us
The company PPH GmbH, founded and located in Erlangen in 2016, is specialized in GMP-consulting and the trade within the medical and pharmaceutical markets. The company assists and consults producers and suppliers of pharmaceuticals in the GMP-market, when it comes to the introduction and/or the actual implementation of pharmaceutical quality systems. Our main building blocks of our consulting services range from feasibility studies and project definition to its implementation. The spectrum of services is completed by qualification, validation and support within GMP-compliant production facilities. The admission of medicinal products and active product ingedients is also part of our consulting services.
Being an international company PPH complies with high quality standards and is experienced in compliance topics and knowledgeable about safety issues.
On the market PPH has focused on trading with equipment for pharmaceutical production facilities. Based on its specialized network with partners, PPH can deliver everything, starting from specific details all the way down to complete technical production services.
Service
We enable you to run your production facility compliant.
The company pph GmbH supports clients within the Life Science industry concerning the implementation of quality requirements according to GMP standards - Good Manufacturing Practice. The main activities consist of professional consulting and the clients' support.
Starting with initial concept studies via qualification and validation up to supervision during routine activities. In short: The support in all questions concerning GMP-compliant production facilities.
Quality-System

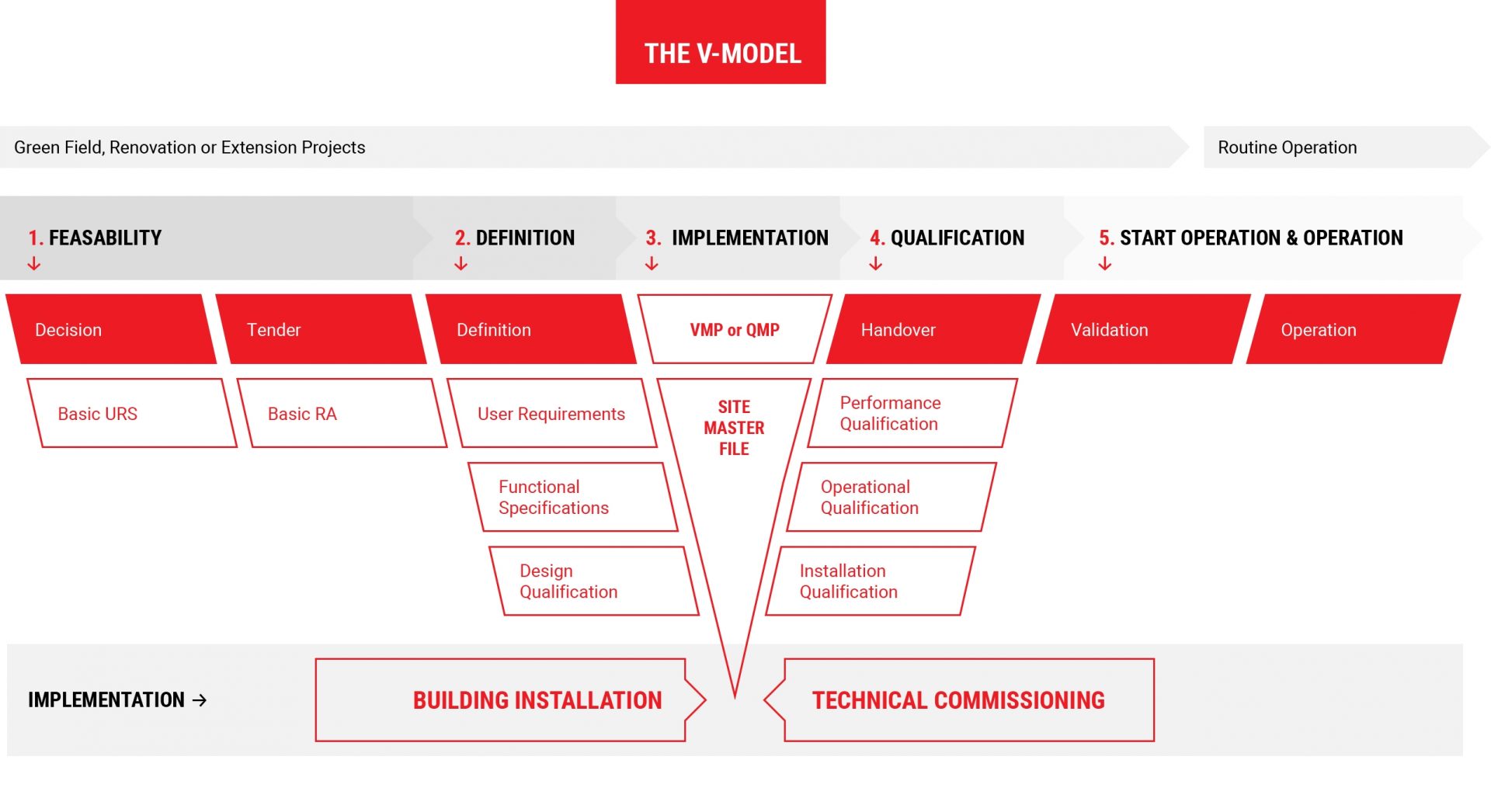
The V-Model applies to all kinds of projects: Green Field Projects, Change and Renovation Projects and Extension Projects. The primary purpose of a quality system is to ensure that adequate quality standards are maintained. You can download a copy of the V-Model here.
-
1.
Feasability
Feasability Study
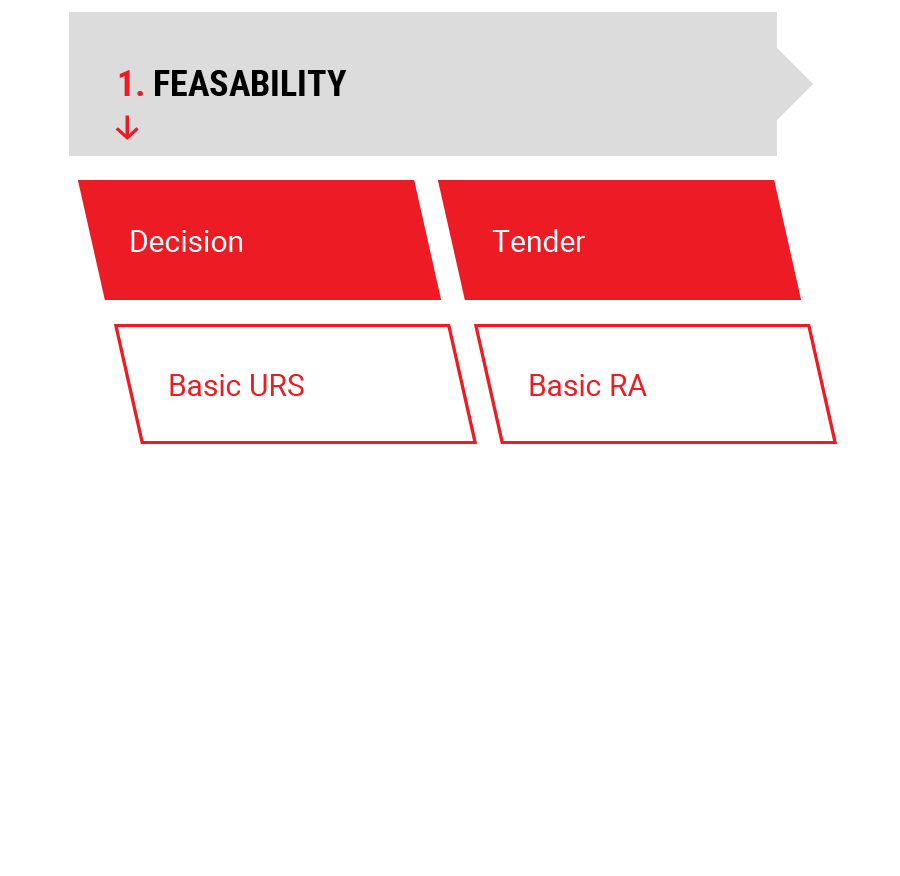
- Project Risk Analysis
- Project User Requirement Specification
- Project Tender Documents
-
2.
Definition
Project Definition
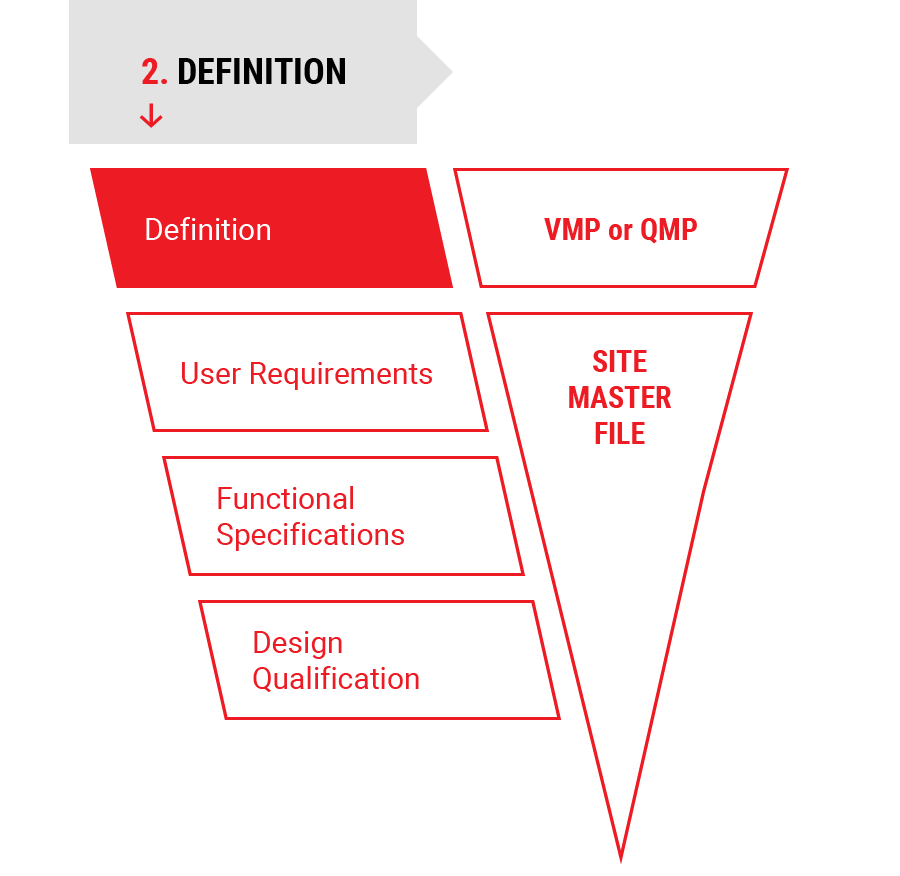
- Validation Master Plan (VMP) or Qualification Master Plan (QMP)
- Site Master File (SMF)
- Risk Analysis (RA)
- User Requirement Specifications (URS)
- Functional Specifications (FS)
- Detailed Design Planning
- Design Qualification (DQ)
- Tender Documents
-
3.
Implementation
Project Implementation
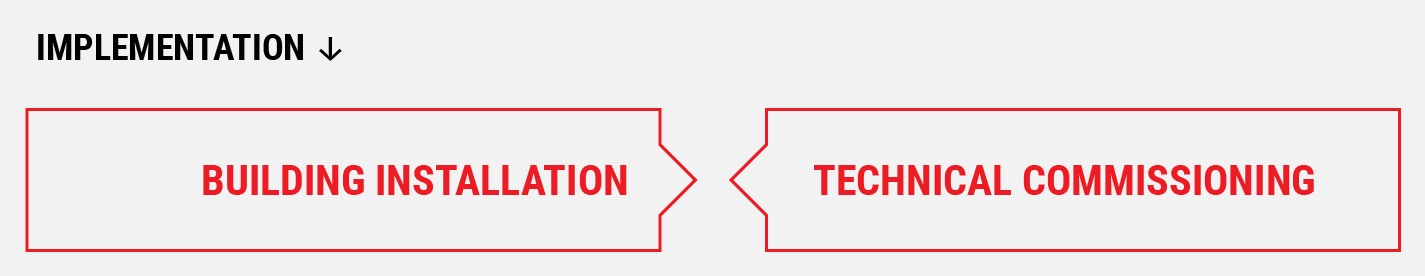
- Support FAT
- Support Assignment
- Support Building and Installation
- Support Technical Commissioning
-
4.
Qualification
Qualification
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Preparation Handover
-
5.
Start Operation
Start Operation & Operation

- Quality Assurance Documents (SOPs)
- Production Documents (SOPs)
- Quality Control Documents (SOPs)
- Documents for Hygiene (SOPs)
- Documents for Precursors (SOPs)
- Product Quality Report (PQR)
- Support during Start of Operation
- Support during Operation
- Support MA
- Support CEP
Projects
We are in the business of Pharmaceutical and Life Science Consulting and are specialized internationally in the field of GMP-compliant qualifications. In addition we are trading with equipment for pharmaceutical production facilities. Our clients rank from clinics and enterprises to universities and research facilities in the markets of medicine, pharma, chemistry and biotechnology. Our goal is to deliver highest quality and reliable structures, and at the end of each project we like to exceed our clients' expectations.
Center of Radiopharmacy at Campus Grosshadern
Construction Project
Constuction of a new laboratories and research center at the Munich campus of Großhadern.
Location
Year
Project Duration
Projektart
Constructed Area
Completion
Within the framework of a PPP projects, the PETNet München GmbH built new laboratories and a research facility to deliver radiopharmaceuticals, located on the property of KUM Munich. The new building is designed to accommodate the aseptic production of sterile pharmaceuticals.
The entire floor space of the planned GMP-areas amounts to 360 m². The production area is divided into two separate sections with production rooms of cleanroom categories D, C, and B. The upper floor of the building contains all administration offices as well as a conference room for the Clinic Großhadern.
Functionally this wing of the building can be reached by a new constructed steel bridge that connects directly into the department of nuclear medicine.
The engaged persons have been Robert Hebel, Helmut Peters and Bernd Perner of perner architects in Rosenheim, co-partner of PPH GmbH.
Heads
Robert Hebel and Helmut Peters look back at a long professional relationship and have decided to establish the enterprise PPH in early 2016. They teamed up more than 10 years ago. Both are linked by the engagement within the niche market of radio isotope production and they pursue the goal to expand the services offered by PPH in the future.

Robert Hebel
General Management
• Several years within Siemens Healthcare as CEO of CT Imaging, PET Net, PET Net Solutions and PET Net München
• General Management Training at London Business School
Requirement Engineering
• More than 10 years experience in several business units of Siemens Healthcare
• Knowledgeable in many requirement engineering methods
• Experience in many direct customer sessions
Marketing
• Placement of several product innovations for Siemens Healthcare
• Placement of syngo software platform strategy
Motivational Leadership
• Experience with large international team
Process Implementation
• Implementation of software quality processes
• Implementation quality systems
Radiopharmacy Consulting
• Design, Construction, Qualification and Validation of complete Radiopharmacy Centers
• Knowledgeable in implementing GMP processes
• Knowledgeable in implementing radiation safety processes
• Experienced in implementing new radio tracers
Robert Hebel studied Physics and Biomedical Engineering at the University in Erlangen, Germany, and obtained his Diploma of Physics in 1984. He has conducted a General Management Training at London Business School.
He spent 23 years with Siemens Medical in Erlangen in different business units (Ultrasound, Computed Tomography, Magnetic Resonance Tomography, PET and Software Development) mostly in the fields of requirement engineering, development and marketing.
While leading a radiopharmaceutical company in Erlangen for seven years he planned and built a green field PET Production Center according to GMP regulations. During his time, he became an expert for a Quality System, Risk Analysis, Requirement Definition, Qualification, Validation in the setting of an aseptic radiopharmaceutical production.
He is co-founder of the company PPH GmbH, Erlangen, Germany, which provides GMP Consulting together with Technology Sourcing.

Helmut Peters
Born in 1959 in Nuremberg, he received his Diploma in Computer Sciences at the Friedrich-Alexander University of Erlangen in 1984. He is skilled in the fields of business administration, management consulting and worked as COO and CFO of owner-managed companies. Especially in the area of whole sale and a supply company for laboratory equipment and consumables he was able to gain detailed experiences. Later on he was responsible to conduct feasibility studies contracted by a company aiming to start a Proton Therapy Center in Erlangen. He also has been in charge of the German branch of an international, stock exchange registered company for orthopedics technology in the fields of finance and accounting, purchasing, controlling and logistics. As of 2009 he managed several subsidiaries of a medicine technical research holding as business administration manager. He also was engaged as CFO of a project company in the framework of a PPP-model in cooperation with the clinical center of the University of Munich. Together with two colleagues, he founded in 2016 the company PPH GmbH.
Contact
PPH GmbH
Burgbergstr. 33B
91054 Erlangen
Phone: +49 173 2596589
Fax: +49 9131 207919
Mail: robert.hebel@pph-gmbh.de
Glossary
- SOP
Standard Operation Procedure
- URS
User Requirement Specification (=tender documents)
- VMP
Validation Master Plan
- OQ
Operational Qualification
- IQ
Installation Qualification
- ICH
International Conference of Harmonization
- ISO
International Organization for Standardization
- PQ
Performance Qualification
- FSD
Functional Specification Document (=user specifications)
- GHTF
Global Harmonization Task Force
- DQ
Design Qualification
- EMEA
European Medicines Agency
- FDA
Food and Drug Agency (US)
- CDRH
Center for Devices and Radiological Health
The terms and definitions in this glossary have been developed by representatives of the International Pharmaceutical Excipients Council (IPEC). The complete glossary and additional information to its origins can be seen at ipec-europe.org ansehen (PDF).
Imprint
PPH GmbH
Burgbergstr. 33B
91054 Erlangen
Phone: +49 173 2596589
Fax: +49 9131 207919
Mail: robert.hebel@pph-gmbh.de
Registergericht Fürth
HRB 15568
Steuernummer 216 / 135 / 30522
UID: DE 305 678 421
Managing Directors: Robert Hebel, Helmut Peters



